Abstract
A sensor for sensitive in situ measurements of carbon monoxide and temperature in combustion gases has been developed using absorption transitions in the (v′=1←v″=0) and (v′=2←v″=1) fundamental bands of CO. Recent availability of mid-infrared quantum-cascade (QC) lasers provides convenient access to the CO fundamental band near 4.7 μm, having approximately 104 and 102 times stronger absorption line-strengths compared to the overtone bands near 1.55 μm and 2.3 μm used previously to sense CO in combustion gases. Spectroscopic parameters of the selected transitions were determined via laboratory measurements in a shock tube over the 1100–2000 K range and also at room temperature. A single-laser absorption sensor was developed for accurate CO measurements in shock-heated gases by scanning the line pair v″=0, R(12) and v″=1, R(21) at 2.5 kHz. To capture the rapidly varying CO time-histories in chemical reactions, two different QC lasers were then used to probe the line-center absorbance of transitions v″=0, P(20) and v″=1, R(21) with a bandwidth of 1 MHz using fixed-wavelength direct absorption. The sensor was applied in successful shock tube measurements of temperature and CO time-histories during the pyrolysis and oxidation of methyl formate, illustrating the capability of this sensor for chemical kinetic studies.

















Similar content being viewed by others
References
M.G. Allen, Meas. Sci. Technol. 9, 545 (1998)
H. Teichert, T. Fernholz, V. Ebert, Appl. Opt. 42, 2043 (2003)
K. Kohse-Höinghaus, R.S. Barlow, M. Aldén, J. Wolfrum, Proc. Combust. Inst. 30, 89 (2005)
R.K. Hanson, Proc. Combust. Inst. 33, 1 (2011)
D.T. Cassidy, L.J. Bonnell, Appl. Opt. 27, 2688 (1988)
R.M. Mihalcea, D.S. Baer, R.K. Hanson, Meas. Sci. Technol. 9, 327 (1998)
B.L. Upschulte, D.M. Sonnenfroh, M.G. Allen, Appl. Opt. 38, 1506 (1999)
M.E. Webber, J. Wang, S.T. Sanders, D.S. Baer, R.K. Hanson, Proc. Combust. Inst. 28, 407 (2000)
J. Wang, M. Maiorov, D.S. Baer, D.Z. Garbuzov, J.C. Connolly, R.K. Hanson, Appl. Opt. 39, 5579 (2000)
V. Ebert, H. Teichert, P. Strauch, T. Kolb, H. Seifert, J. Wolfrum, Proc. Combust. Inst. 30, 1611 (2005)
X. Chao, J.B. Jeffries, R.K. Hanson, Meas. Sci. Technol. 20, 115201 (2009)
R.K. Hanson, P.A. Kuntz, C.H. Kruger, Appl. Opt. 16, 2045 (1977)
R.K. Hanson, P.K. Falcone, Appl. Opt. 17, 2477 (1978)
M. Schoenung, R.K. Hanson, Combust. Sci. Technol. 24, 227 (1981)
J.H. Miller, S. Elreedy, B. Ahvazi, F. Woldu, P. Hassanzadeh, Appl. Opt. 32, 6082 (1993)
R. Barron-Jimenez, J.A. Caton, T.N. Anderson, R.P. Lucht, T. Walther, S. Roy, M.S. Brown, J.R. Gord, Appl. Phys. B 85, 185 (2006)
A.A. Kosterev, F.K. Tittel, R. Köhler, C. Gmachl, F. Capasso, D.L. Sivco, A.Y. Cho, S. Wehe, M.G. Allen, Appl. Opt. 41, 1169 (2002)
J. Vanderover, M.A. Oehlschlaeger, Appl. Phys. B 99, 353 (2010)
F. Capasso, R. Paiella, R. Martini, R. Colombelli, C. Gmachl, T.L. Myers, M.S. Taubman, R.M. Williams, C.G. Bethea, K. Unterrainer, H.Y. Hwang, D.L. Sivco, A.Y. Cho, A.M. Sergent, H.C. Liu, E.A. Whittaker, IEEE J. Quantum Elect 38, 511 (2002)
A. Kosterev, G. Wysocki, Y. Bakhirkin, S. So, R. Lewicki, M. Fraser, F. Tittel, R.F. Curl, Appl. Phys. B 90, 165 (2008)
R.F. Curl, F. Capasso, C. Gmachl, A.A. Kosterev, B. McManus, R. Lewicki, M. Pusharsky, G. Wysocki, F.K. Tittel, Chem. Phys. Lett. 487, 1 (2010)
V. Nagali, S.I. Chou, D.S. Baer, R.K. Hanson, J. Segall, Appl. Opt. 35, 4026 (1996)
G. Birnbaum, in Adv. Chem. Phys, ed. by J.O. Hirschfelder. Intermolecular forces, vol. 12 (Interscience, New York, 1967)
X. Zhou, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 81, 711 (2005)
W. Ren, D.F. Davidson, R.K. Hanson, Int. J. Chem. Kinet. doi:10.1002/kin.20599 (2012)
R.C. Millikan, D.R. White, J. Chem. Phys. 39, 3209 (1963)
J.-P. Bouanich, C. Haeusler, J. Quant. Spectrosc. Radiat. Transfer 12, 695 (1972)
P.L. Varghese, R.K. Hanson, J. Quant. Spectrosc. Radiat. Transfer 24, 479 (1980)
D.F. Davidson, Z. Hong, G.L. Pilla, A. Farooq, R.D. Cook, R.K. Hanson, Proc. Combust. Inst. 33, 151 (2011)
S. Dooley, M.P. Burke, M. Chaos, Y. Stein, F.L. Dryer, V.P. Zhukov, O. Finch, J.M. Simmie, H.J. Curran, Int. J. Chem. Kinet. 42, 527 (2010)
R.J. Kee, F.M. Ruply, J.A. Miller, Chemkin Collection (Reaction Design, Inc., San Diego, 2010)
Acknowledgements
This work was supported by the Combustion Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001198, the Army Research Office (ARO) with Dr. Ralph Anthenien as contract monitor, and the Air Force Office of Scientific Research (AFOSR) with Dr. Julian Tishkoff as technical monitor. The authors thank Dr. Jay Jeffries for his help on the selection and specification of the lasers and acquisition of the needed support electronics.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
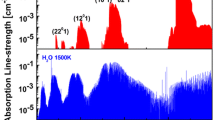
High-resolution absorption spectroscopy of the CO transition v″=1, R(22) at 2194.46 cm−1 has been recorded in high temperature shock tube experiments (0.496 % CO/1 % H2/Ar, hydrogen is added to accelerate CO vibrational relaxation). A representative line-shape for the R(22) transition at 2162 K, 1.3 atm is illustrated in Fig. 18. It is found that R(22) is blended with a weak, nearby transition v″=2, R(32) centered at 2194.44 cm−1. Our observation is also proved by fitting the absorption data using the Voigt profile as shown in Fig. 19. The one-line Voigt fit gives a peak-normalized residual of 10 %, compared to 1.8 % using the two-line Voigt fit. This unknown transition is probably coming from an isotope of CO in the mixture.
One-line and two-line best-fit Voigt profiles for the absorption data in Fig.18. The residual of the fits are shown in the lower panels
Rights and permissions
About this article
Cite this article
Ren, W., Farooq, A., Davidson, D.F. et al. CO concentration and temperature sensor for combustion gases using quantum-cascade laser absorption near 4.7 μm. Appl. Phys. B 107, 849–860 (2012). https://doi.org/10.1007/s00340-012-5046-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5046-1